Metal-free three-component cyanoalkylation of quinoxalin-2(1H)-ones with vinylarenes and azobis(alkylcarbonitrile)s†
Abstract
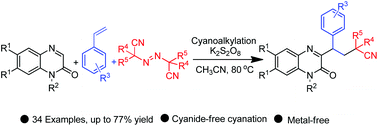
We have unveiled an efficient synthesis of cyanoalkylated quinoxalin-2(1H)-ones via a three-component radical cascade reaction of quinoxalin-2(1H)-ones with vinylarenes and azobis(alkylcarbonitrile)s. K2S2O8 takes part in the reaction as a sole oxidant under base, additive, and metal-free conditions, producing the three-component products in moderate to good yields. The protocol also works with phenylacetylene in the absence of vinyl arenes and provides the respective product. Furthermore, different control experiments with radical scavengers like 2,2,6,6-tetramethylpiperidine-1-oxyl and diphenyl ethylene prove the generation of radicals during the reaction.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...