Nicked Invader probes: multistranded and sequence-unrestricted recognition of double-stranded DNA†
Abstract
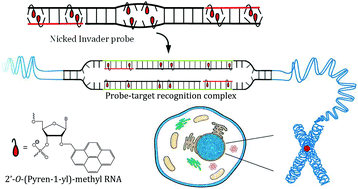
Major efforts have been devoted to the development of constructs that enable sequence-specific recognition of double-stranded (ds) DNA, fueled by the promise for enabling tools for applications in molecular biology, diagnostics, and medicine. Towards this end, we have previously introduced Invader probes, i.e., short DNA duplexes with +1 interstrand zipper arrangements of intercalator-functionalized nucleotides. The individual strands of these labile probes display high affinity towards complementary DNA (cDNA), which drives sequence-unrestricted dsDNA-recognition. However, recognition of long targets is challenging due to the high stability of the corresponding probes. To address this, we recently introduced toehold Invader probes, i.e., Invader probes with 5′-single-stranded overhangs. The toehold architecture allows for shorter double-stranded segments to be used, which facilitates probe dissociation and dsDNA-recognition. As an extension thereof, we here report the biophysical and dsDNA-targeting properties of nicked Invader probes. In this probe architecture, the single-stranded overhangs of toehold Invader probes are hybridized to short intercalator-modified auxiliary strands, leading to formation of additional labile segments. The extra binding potential from the auxiliary strands imparts nicked Invader probes with greater dsDNA-affinity than the corresponding toehold or blunt-ended probes. Recognition of chromosomal DNA targets, refractory to recognition by conventional Invader probes, is demonstrated for nicked Invader probes in the context of non-denaturing FISH experiments, which highlights their utility as dsDNA-targeting tools.


 Please wait while we load your content...
Please wait while we load your content...