Ultrafast synthesis of SiC nanowire webs by floating catalysts rationalised through in situ measurements and thermodynamic calculations†
Abstract
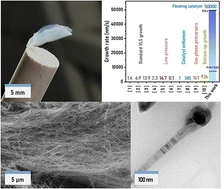
This work presents the synthesis of SiC nanowires floating in a gas stream through the vapour–liquid–solid (VLS) mechanism using an aerosol of catalyst nanoparticles. These conditions lead to ultrafast growth at 8.5 μm s−1 (maximum of 50 μm s−1), which is up to 3 orders of magnitude above conventional substrate-based chemical vapour deposition. The high aspect ratio of the nanowires (up to 2200) favours their entanglement and the formation of freestanding network materials consisting entirely of SiCNWs. The floating catalyst chemical vapour deposition growth process is rationalised through in situ sampling of reaction products and catalyst aerosol from the gas phase, and thermodynamic calculations of the bulk ternary Si–C–Fe phase diagram. The phase diagram suggests a description of the mechanistic path for the selective growth of SiCNWs, consistent with the observation that no other types of nanowires (Si or C) are grown by the catalyst. SiCNW growth occurs at 1130 °C, close to the calculated eutectic. According to the calculated phase diagram, upon addition of Si and C, the Fe-rich liquid segregates a carbon shell, and later enrichment of the liquid in Si leads to the formation of SiC. The exceptionally fast growth rate relative to substrate-based processes is attributed to the increased availability of precursors for incorporation into the catalyst due to the high collision rate inherent to this new synthesis mode.


 Please wait while we load your content...
Please wait while we load your content...