Sequential galvanic replacement mediated Pd-doped hollow Ru–Te nanorods for enhanced hydrogen evolution reaction mass activity in alkaline media†
Abstract
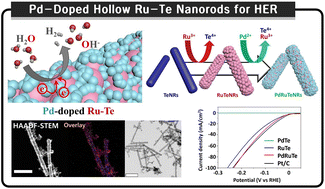
High catalytic activity, long-term stability, and economical Pt-free catalysts for the hydrogen evolution reaction (HER) are required for the conversion of renewable energy systems. Noble nanomaterial Pt is a superior electrolysis catalyst for water splitting under typical experimental conditions with a relatively low overpotential. However, the use of Pt is limited by its high cost and activity degradation over time. Among several prospective alternatives, Ru has emerged as a promising alkaline electrolysis catalyst because of its significant catalytic activity and reduced cost compared to Pt. We designed and suggested Pd-doped hollow Ru–Te nanorods (PdRuTeNRs) via successive galvanic replacement reactions of sacrificial Te nanotemplates to further boost efficiency. The Pd/partially oxidized RuO2/Ru/Te hetero-interfaced composition exhibited an HER mass activity of 11.3 A g−1 Ru, twice that of Pt. In addition, the present PdRuTeNRs sufficiently maintained the activity from the 2000-cycle continuous test, greatly reducing the required cost by a quarter.


 Please wait while we load your content...
Please wait while we load your content...