Abstract
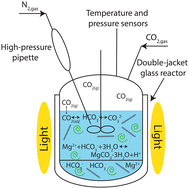
Carbonate precipitation, as part of the carbon dioxide (CO2) mineralization process, is generally regarded as a high-temperature, high-pressure, and high-purity CO2 process. Typical conditions consist of temperatures around 120 °C and a pressure of 100 bar of pure CO2, making the process costly. A major challenge facing carbonate precipitation is performing the reaction at low temperatures and low partial pressures of CO2 (pCO2) such as 25 °C and CO2 flue gas concentration. In this work, we investigated the effect of carbonic anhydrase (CA) to favor magnesium (Mg) carbonate precipitation at low temperatures and low pCO2. CA is an enzyme that accelerates CO2 hydration promoting its conversion into HCO3− and then CO32−. This increases supersaturation with respect to Mg-carbonates. A geochemical model was implemented and used to identify supersaturated conditions with respect to Mg-carbonates. Tests were run at 25, 40, and 50 °C and at 1 bar of either pure CO2 or 10 vol% CO2 and 90 vol% N2. The concentration of 10 vol% CO2 was chosen to resemble CO2 concentration in flue gas. In selected tests, the CA enzyme was added directly as bovine CA or through microalgae (Scenedesmus obliquus). Experiments were run for 48 hours; 24 hours to reach equilibrium, then another 24 hours until the supersaturated conditions were established. After 48 hours the experiments were interrupted and the solids were characterized. Results show that the addition of CA, either directly or through Scenedesmus obliquus, enhances Mg-carbonate precipitation. Regardless of the temperature, the precipitates were made entirely of nesquehonite (MgCO3-3H2O) when pure CO2 was used. Otherwise, a solid solution containing brucite (Mg(OH)2) and MgCO3-3H2O was formed. Overall, these findings suggest that CA can promote carbonate precipitation at low temperatures, pressures, and CO2 purity. The enzyme is effective when added directly or supplied through microalgae, opening up the possibility for a CO2 mineralization process to be implemented directly at a combustion plant as a CO2 storage option without preliminary CO2 capture.
- This article is part of the themed collection: CO2 capture and conversion


 Please wait while we load your content...
Please wait while we load your content...