Designing 1D multiheme peptide amphiphile assemblies reminiscent of natural systems†
Abstract
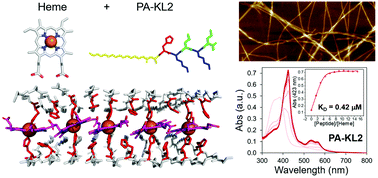
Protein assemblies that bind and organize ordered arrays of cofactors yield function structures. Multiheme assemblies found in nature yield electronically conductivity 1D nanoscale fibers and are employed in anaerobic respiration. To understand the fundamental characteristics of these organized arrays, the design of peptide amphiphiles that assemble into 1D nanostructures and yield metalloporphyrin binding sites is presented. One challenge with this class of peptide amphiphiles is identifying the correct sequence composition for high affinity binding with high heme density. Here, the peptide c16-AH(Kx)n-CO2H is explored to identify the impact of sequence length (n) and amino acid identity (x = L, I, or F) on binding affinity and midpoint potential. When n = 2, the peptide assembly yields the greatest affinity. The resulting nanoscale assemblies yield ordered arrays of the redox active molecule heme and have potential utility in the development of supramolecular bioelectronic materials useful in sensing as well as the development of enzymatic materials.


 Please wait while we load your content...
Please wait while we load your content...