Theory for nanoscale curvature induced enhanced inactivation kinetics of SARS-CoV-2
Abstract
We develop a novel theory for the nanomorphology dependent outer sphere heterogeneous electron transfer (ET) rate constant (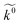 ) based on an energy level alignment approach.
) based on an energy level alignment approach.  is modelled through the activation free energy, which is a product of the water monolayer covered metal work function (WF) and the fractional electronic charge exchanged at the transition state (attained through the alignment of the metal Fermi and HOMO/LUMO energy levels of the electroactive species). The theory shows that
is modelled through the activation free energy, which is a product of the water monolayer covered metal work function (WF) and the fractional electronic charge exchanged at the transition state (attained through the alignment of the metal Fermi and HOMO/LUMO energy levels of the electroactive species). The theory shows that  is an exponentially increasing and decreasing function of the mean curvature in concave and convex nanomorphologies, respectively, for electroactive species or proteins involving their HOMO energy. For the specific spike protein of SARS-CoV-2, we have estimated the half lifetime (t1/2) and degree of inactivation as a function of the metal WF, nanostructure mean curvature, spike protein HOMO energy, and the environmental temperature (T). By varying the metal from Ag to Au, t1/2 is reduced from 7 h to 4 min, respectively. The reduction in the copper nanoparticle size from 50 to 5 nm increases the degree of inactivation from 60 to 99.6% (with a reduction factor of 10 in t1/2). Similarly, the increase in T from 10 °C to 65 °C causes a 100 times lowering of the t1/2 and t99.9% of SARS-CoV-2 on Cu metal. The theory predicts that
is an exponentially increasing and decreasing function of the mean curvature in concave and convex nanomorphologies, respectively, for electroactive species or proteins involving their HOMO energy. For the specific spike protein of SARS-CoV-2, we have estimated the half lifetime (t1/2) and degree of inactivation as a function of the metal WF, nanostructure mean curvature, spike protein HOMO energy, and the environmental temperature (T). By varying the metal from Ag to Au, t1/2 is reduced from 7 h to 4 min, respectively. The reduction in the copper nanoparticle size from 50 to 5 nm increases the degree of inactivation from 60 to 99.6% (with a reduction factor of 10 in t1/2). Similarly, the increase in T from 10 °C to 65 °C causes a 100 times lowering of the t1/2 and t99.9% of SARS-CoV-2 on Cu metal. The theory predicts that  involving the HOMO energy level of a protein follows the surface nanostructure shape dependent order as follows: spherical nanoparticle > cylindrical nanorod > cylindrical nanopore > spherical nanocavity, while the opposite trend is observed in the case of the LUMO energy level participation. Finally, the theory shows agreement with the reported experimental data of the degree of inactivation of SARS-CoV-2 on Ag and Cu nanoparticles.
involving the HOMO energy level of a protein follows the surface nanostructure shape dependent order as follows: spherical nanoparticle > cylindrical nanorod > cylindrical nanopore > spherical nanocavity, while the opposite trend is observed in the case of the LUMO energy level participation. Finally, the theory shows agreement with the reported experimental data of the degree of inactivation of SARS-CoV-2 on Ag and Cu nanoparticles.



 Please wait while we load your content...
Please wait while we load your content...