Putting together the puzzle of ion transfer in single-digit carbon nanotubes: mean-field meets ab initio†
Abstract
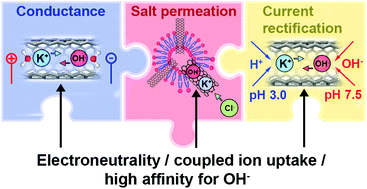
Nature employs channel proteins to selectively pass water across cell membranes, which inspires the search for bio-mimetic analogues. Carbon nanotube porins (CNTPs) are intriguing mimics of water channels, yet ion transport in CNTPs still poses questions. As an alternative to continuum models, here we present a molecular mean-field model that transparently describes ion coupling, yet unlike continuum models, computes ab initio all required thermodynamic quantities for the KCl salt and H+ and OH− ions present in water. Starting from water transfer, the model considers the transfer of free ions, along with ion-pair formation as a proxy of non-mean-field ion–ion interactions. High affinity to hydroxide, suggested by experiments, making it a dominant charge carrier in CNTPs, is revealed as an exceptionally favorable transfer of KOH pairs. Nevertheless, free ions, coexisting with less mobile ion-pairs, apparently control ion transport. The model well explains the observed effects of salt concentration and pH on conductivity, transport numbers, anion permeation and its activation energies, and current rectification. The proposed approach is extendable to other sub-nanochannels and helps design novel osmotic materials and devices.


 Please wait while we load your content...
Please wait while we load your content...