Sulfur-treated TiO2 shows improved alcohol dehydration activity and selectivity†
Abstract
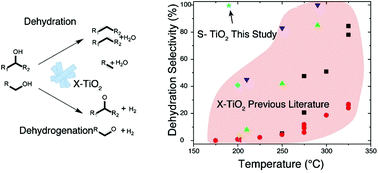
The dehydration of alcohols is an important class of reactions for the development of fossil-free fuel and chemical industries. Acid catalysts are well known to enhance the reactivity of alcohols following two main pathways of either dehydration to olefins or dehydrogenation to ketones/aldehydes. TiO2 surfaces have been well documented for primary and secondary alcohol dehydration with selectivity ranging from 1–100% towards dehydration products based on process conditions and catalyst structure. In this work we document the effects of various sulfur treatments of TiO2 surfaces which induce higher activity and, more importantly, higher selectivity for alcohol dehydration than untreated surfaces. The increase in activity and >99% dehydration selectivity is coupled with demonstrated stability for several hours on stream at high conversion. Using temperature programmed reaction studies, XPS and FT-IR spectroscopy, we identify Lewis acidic sites correlated with sulfate species on TiO2 surfaces as active sites for the reaction.


 Please wait while we load your content...
Please wait while we load your content...