Photocatalytic hydrogen generation from a methanol–water mixture in the presence of g-C3N4 and graphene/g-C3N4†
Abstract
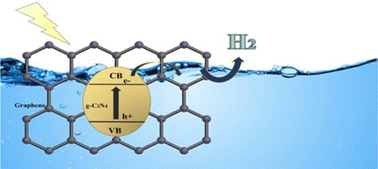
Graphitic carbon nitride (g-C3N4), possessing high thermal and chemical stability, non-toxicity, facile synthesis, and low band gap energy is a promising candidate for photocatalytic applications. In this study, bulk and exfoliated g-C3N4 were synthesised from different precursors (melamine and urea). Moreover, the surface of bulk g-C3N4 and exfoliated g-C3N4 was modified with graphene (0.5 wt% and 1 wt%) aiming to obtain a prolonged carrier lifetime. The effect of g-C3N4 synthesis from various precursors and the influence of graphene content on the photocatalytic activity during the degradation of a water–methanol mixture under UVC irradiation were examined, in comparison to commercial P25. Hydrogen, methane and carbon monoxide were the decomposition products; hydrogen was the main product of decomposition, whereas CH4 and CO resulting from the reduction of CO2 were generated in a significantly smaller amount. All the produced photocatalysts, whether pure or modified with graphene, exhibited higher activity than the commercial photocatalyst P25.


 Please wait while we load your content...
Please wait while we load your content...