Understanding the regioselectivity of 5-substituted 1H-tetrazoles alkylation†
Abstract
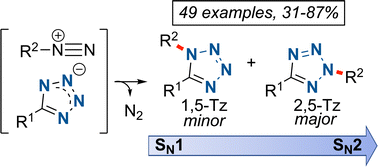
The synthesis of disubstituted tetrazoles is described from 1H-5-monosubstituted tetrazoles via the aliphatic amine diazotization reaction, which forms a transient alkyl diazonium intermediate, acting as an alkylating agent. Although the 2,5-disubstituted tetrazole (2,5-Tz) is preferentially formed in moderate to excellent yields, it is also possible to isolate the minor 1,5-disubstituted tetrazole (1,5-Tz) in several cases. The regioselectivities (1,5-Tz:2,5-Tz) are highly variable and cannot be exclusively attributed to the steric hindrance of the electrophile. A new rationale to explain the observed regioselectivity, based on the difference in mechanism between first- and second-order nucleophilic substitutions, is thus proposed. In addition, in some cases the intramolecular stabilization of the resulting diazonium influences the regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...