Improving the yield of graphene oxide-catalysed N-heterocyclization of amines through fed batch mode†
Abstract
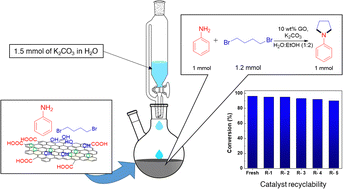
The use of graphene oxide, a metal-free, heterogeneous carbocatalyst for a facile, efficient, and simple protocol for N-heterocyclization of aromatic amines with dihaloalkane to give azacycloalkanes and isolindolines was studied. The catalyst displays excellent selectivity and a high yield in a short reaction time under mild reaction conditions of temperature and at atmospheric pressure in the presence of H2O:EtOH, green solvents. GO with oxygen functionality serves a dual purpose; it provides an anchor site to the reactant and serves as a co-catalyst initiator by aiding base dissociation. The reaction of aromatic amines with dihaloalkanes was optimized for catalyst loading, base, base loading, reaction temperature, and solvents to give 84% yield at 80 °C, in 1 : 2 H2O : EtOH solvent within 4 h. Yield improvement was observed on performing the reaction in fed-batch mode. The reaction protocol was further modified to employ a fed-batch strategy to maintain low concentration of base and minimize the inactivation of the catalyst. The nominal yield of 40% was improved 2.3-fold employing a fed-batch strategy. The GO catalyst was easily separated by filtration and showed remarkable recyclability up to 5 cycles. Different substituents of amines and dihaloalkanes were studied and characterized by 1H and 13C NMR. The mild reaction conditions and readily re-usable nature of the GO catalyst show immense potential for application of the methodology in synthetic strategies for the production of pharmaceutical intermediates and APIs.

 Please wait while we load your content...
Please wait while we load your content...