Hydrolytic dehydrogenation of ammonia borane in neat water using recyclable zeolite-supported cyclic alkyl amino carbene (CAAC)–Ru catalysts†
Abstract
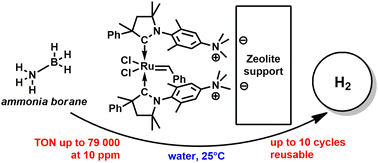
Cyclic alkyl amino mono- and bis-carbene ruthenium (CAAC–Ru) complexes were immobilized on mesoporous Y zeolite (catalysts 3 and 4) and showed high activity and stability in ammonia borane (AB) hydrolytic dehydrogenation. Both catalysts have a Ru content as low as 0.1 wt%. Catalysts 3 and 4 provide a reasonable activity even at a loading of 10 ppm (0.001 mol%) giving a turnover number (TON) of 79 000 molH2 molcat−1. The optimal loading, however, was found to be slightly higher for catalyst 4, at around 50 ppm (0.005 mol%), giving a turnover frequency (TOF) of 8500 molH2 molcat−1 h−1 and a TON of 49 375 molH2 molcat−1 whilst retaining a high nH2/nAB ratio (2.51). This value is higher than those observed for its homogeneous analogue 2 (TOF, 7500 molH2 molcat−1 h−1; TON, 43 600 molH2 molcat−1; released nH2/nAB ratio, 2.18). Interestingly, it was found that the zeolite-supported catalyst gave a better performance than the non-supported water-soluble derivatives. No ruthenium leaching was detected for any of the zeolite-supported systems. Catalyst 4 showed a significantly higher activity than catalyst 3 and could be recycled up to 10 times. Catalyst 4 demonstrated a reasonable hydrolytic dehydrogenation activity even after three days in water upon exposure to air. The highest TON (79 000 molH2 molcat−1) obtained with catalyst 4 is equal to 1.68 kg H2 per gram of ruthenium metal.


 Please wait while we load your content...
Please wait while we load your content...