Theoretical study on the reaction mechanism of Si2Cl6 and HCl catalyzed by amine catalysts†
Abstract
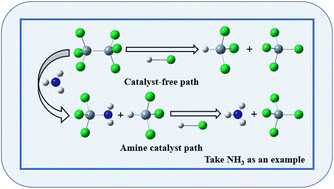
The reaction of Si2Cl6 and HCl to form SiHCl3 and SiCl4 catalyzed with aliphatic and aromatic amines was explored in detail by density functional theory calculations. All stagnation points included in the reaction path were estimated at the WB97XD/6-311++G(2d,p) level. Without a catalyst, Si2Cl6 reacts with HCl to form SiHCl3 and SiCl4 through only one transition state. The activation energy barrier for this process is 245.29 kJ mol−1. The amine catalysts alter the reaction pass from one transition state to two transition states. The rate-determining step is that amine attacks Si2Cl6 to yield SiHCl3 and Amino-SiCl3 intermediate (the activation energy barrier is within 98.27–166.37 kJ mol−1). The next step is that the amino-SiCl3 intermediate reacts with HCl to produce SiCl4 and recycle the amine catalyst. The catalyst significantly reduces the activation energy barrier of the reaction, and aliphatic amines are more efficient than aromatic amines. We believe that the results of our calculations and discussions are vital to better understand how the reactions occur and to guide the development of catalysts.


 Please wait while we load your content...
Please wait while we load your content...