Metal-free synthesis of secondary amides using N-Boc-O-tosylhydroxylamine as nitrogen source via Beckmann rearrangement†
Abstract
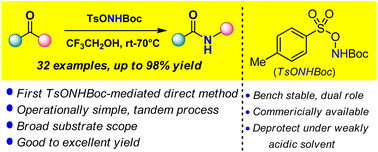
Herein, we report the first direct method for the synthesis of secondary amides from ketones via the Beckmann rearrangement using N-Boc-O-tosylhydroxylamine (TsONHBoc) as the aminating agent. This reagent is expected to play a dual role, first in the formation of the activated oxime intermediate, and then via facilitation of the amide formation as a Brønsted acid by the tosylic acid by-product. The metal and additive-free one-step method progresses in TFE solvent through the in situ generation of the primary amine reagent (TsONH2), and produces a water-soluble by-product.


 Please wait while we load your content...
Please wait while we load your content...