Base-induced isomerization of red uroleuconaphins revisited: characterization and absolute stereochemistry of the yellow aphid pigments uroleuconaphins A2 and B2†
Abstract
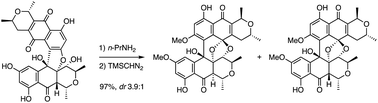
We present herein the base-induced isomerization of red uroleuconaphins A1 and B1 to yellow uroleuconaphins A2 and B2, respectively. Based on our preliminary results, we optimized the conversion conditions by the extensive screening of bases to give the desired products in high yield. These yellow pigments were dimethylated using TMSCHN2 for stabilization, thereby enabling the separation of the diastereomers at C10a. The absolute stereochemistry of the yellow uroleuconaphins was determined by comparison with natural isolated samples.


 Please wait while we load your content...
Please wait while we load your content...