A two-dimensional zeolitic imidazolate framework loaded with an acrylate-substituted oxoiron cluster as an efficient electrocatalyst for the oxygen evolution reaction†
Abstract
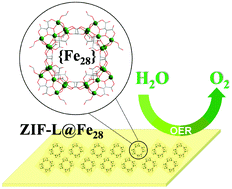
An acrylate-substituted oxoiron cluster {Fe28}, with acidity and poor solubility, has been loaded with 2D zeolitic imidazolate frameworks (ZIF)-L. The obtained composite ZIF-L@Fe28 (120 mg) affords the best oxygen evolution reaction (OER) activity and stability with an overpotential of 312 mV at 10 mA cm−2, and a Tafel slope of 78 mV dec−1, compared with ZIF-L@Fe28 (80, 160 mg), ZIF-67@Fe28, the pristine 2D ZIF-L and 3D ZIF-67. These controls suggest that the superior performance is attributable to the {Fe28} ↔ ZIF bidirectional synergistic effect and the etching effect of {Fe28} in aqueous solution on the ZIF-L. Therefore, we propose that the strategy of preparing ZIF composites loaded with polynuclear metal-oxo clusters fully composed of OER-active metal sites (Fe, Co and Ni) may open up new avenues for the design of novel non-noble electrocatalysts for the OER.


 Please wait while we load your content...
Please wait while we load your content...