On the usability of salt metathesis reactions for the synthesis of sterically crowded tris-formamidinate Ln(iii) complexes: success and limits. Spontaneous reduction of Eu(iii) to Eu(ii)†
Abstract
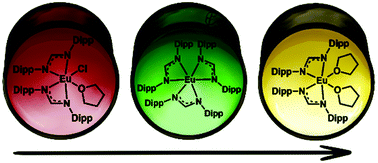
Homoleptic [Ln(FormDipp)3] (FormDipp− = CH(NDipp)2− (Dipp = 2,6-diisopropylphenyl), Ln = Nd, Eu) complexes bearing three bulky formamidinate ligands were successfully obtained using the salt metathesis approach, viz. the reaction of [Ln(FormDipp)2Cl(thf)n] (Ln = Nd (n = 2), Ln = Eu (n = 1)) with K(FormDipp) in toluene at 100 °C. This method is not useful for the Ln smaller than Eu: all attempts to realize it for Gd and Tb were unsuccessful. In the case of Eu, the tris-formamidinate complex was obtained in toluene, but in a thf/toluene mixture, a spontaneous reduction of Eu(III) to Eu(II) leading to the formation of [Eu(FormDipp)2(thf)2] was observed. The Eu(III) to Eu(II) conversion is rather sterically induced. UV-Vis spectroscopy monitoring of the reaction indicates it to be a zero-order process. Possible routes of the reaction are proposed.


 Please wait while we load your content...
Please wait while we load your content...