Design, synthesis and biological evaluation of 4-phenoxy-pyridine/pyrimidine derivatives as dual VEGFR-2/c-Met inhibitors†
Abstract
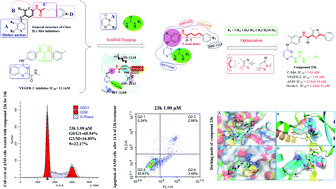
A class of 4-phenoxy-pyridine/pyrimidine derivatives (23a–23p and 24a–24h) were designed, synthesized and evaluated as potent dual VEGFR-2/c-Met inhibitors. The in vitro anti-cancer cell proliferative activity of the compounds indicated that compound 23k was regarded as a promising derivative. Compared to the lead compounds Foretinib and Sorafenib, 23k showed excellent inhibitory activity against the A549, MCF-7, HepG2 and Ovcar-3 cell lines with IC50 values of 2.16 ± 0.19 μM, 9.13 ± 0.65 μM, 20.15 ± 2.64 and 9.65 ± 0.51 μM, respectively. In addition, 23k exhibited low toxicity to human normal cells (LO2 cells) with IC50 values above 100 μM. In kinase assays, the most promising compound 23k showed excellent kinase inhibitory activity and selectivity against VEGFR-2 and c-Met with IC50 values of 1.05 and 1.43 μM, respectively. Further activity studies demonstrated that 23k not only induced apoptosis in A549, but also blocked the A549 cell lines in the G0/G1 phase in a dose-dependent manner. Moreover, molecular docking and molecular dynamics simulation studies revealed the binding modes of 23k to VEGFR-2 and c-Met. The binding modes and structure–activity relationship (SAR) investigations of synthetic pyridine/pyrimidine derivatives, as well as a brief mechanism of their anti-VEGFR-2 and c-Met kinase activities, are presented in this paper.


 Please wait while we load your content...
Please wait while we load your content...