Anisotropic dye adsorption using templated smectic nanoporous polymer films: effects of pore size and pore charges on the adsorption†
Abstract
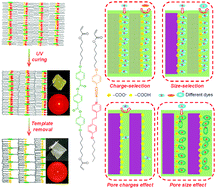
Selective 2-dimensional (2D) nanoporous polymer films have been developed using a templating method based on hydrogen-bonding supramolecular liquid crystals (LCs) containing benzoic acid and pyridine groups (6OBA·NC6·C6H). The smectic lamellar structures are maintained and anionic nanopores are obtained after template (NC6) removal. The NC6-removed polymer films demonstrate anisotropic adsorption toward cationic dye methylene blue (MB) with a high adsorption capacity of 688 mg g−1 under planar alignment. The specific pore dimension with anionic interior shows size- and charge-selectivity. The effects of pore size and pore charges on MB adsorption are investigated using the NC6-removed films with extra pores or non-charged pores, respectively. 5CB is used as the intermolecular template to produce extra nanopores. The obtained results show that the equilibrium adsorption capacity (qe) of MB onto the NC6/5CB template-removed film (T-removed film) does not increase evidently. Non-charged pores generated from the hydrochloric acid (HCl) treatment of the NC6-removed film show poor dye adsorption, indicating that pore charges strongly affect MB adsorption because carboxylate groups act as the active adsorption sites and electrostatic interactions contribute to MB adsorption. Adsorption kinetics can be well described using the pseudo-second-order kinetic model. Also, satisfactory regenerability of the functional polymer films can be achieved by simple HCl treatment and ethanol washing. Such functional polymer films with desired pore dimensions and pore charges can serve as excellent adsorbents to effectively remove cationic contaminants from polluted water.


 Please wait while we load your content...
Please wait while we load your content...