Exploring the membrane fluidity of phenyl boronic acid functionalized polymersomes using the FRAP technique and their application in the pH-sensitive release of curcumin†
Abstract
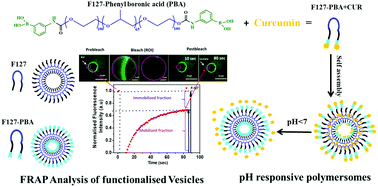
Although the functionalization of drug-delivery systems (DDSs) for targeting tumour cells has been reported earlier, the changes in the membrane fluidity due to functionalization have not yet been well studied. Here, we report an experimental study into the alterations in the membrane fluidity brought about by the functionalization of the polymer and subsequent preparation of giant polymersomes. Polymer-based nanocarriers, such as polymersomes, represent one of the emerging classes of DDSs. They are known to be versatile carriers due to their long blood circulation time, colloidal stability, tunable membrane properties and ability to encapsulate hydrophobic and hydrophilic drugs similar to liposomes. Their clinical success relies on controlled drug targeting, surface functionalization and their physicochemical properties. In this study, two triblock (PEO–PPO–PEO) copolymers composed of hydrophilic polyethylene oxide (PEO) and hydrophobic polypropylene oxide (PPO) polymer units with different molecular weights were selected. These amphiphile polymers could be assembled into giant unilamellar vesicles due to their large PPO contents. The contribution of the PPO units in the membrane bilayer was studied in detail. Further, the polymers were functionalized with an active tumour-targeting ligand, namely, phenyl boronic acid (PBA), to study the role of functionalization on the membrane fluidity using the ‘fluorescence recovery after photobleaching (FRAP)’ technique. Finally, we demonstrated the PBA receptor targeted pH-sensitive release of curcumin (CUR) using these functionalized vesicles.


 Please wait while we load your content...
Please wait while we load your content...