Unexpected ester and phosphonate radical generation by hypervalent iodine compounds for synthesizing 6-phenanthridine derivatives†
Abstract
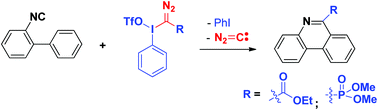
An unprecedented elimination of diazomethane process of a diazo group-bearing hypervalent iodine reagent has been described. This process provides ester and phosphonate radicals, which can react with 2-isocyanobiaryls to afford phenanthridine-6-carboxylate/phosphonate derivatives in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...