μ-Oxo-bridged iron(iii) complexes for the selective reduction of aromatic ketones catalyzed through base promoted in situ nanoparticle formation†
Abstract
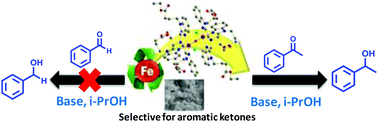
Transfer hydrogenation of ketones is an important method to produce alcohols for various industrial applications. Herein, we described the synthesis of two new μ-oxo-bridged diiron complexes 1 and 2 having nitrogen donors only, and tested their application in the transfer hydrogenation (TH) of aromatic ketones using isopropyl alcohol (i-PrOH) as a hydrogen donor. The identities of these complexes were confirmed using various analytical and spectroscopic evidence. Moreover, the structure of complex 1 was confirmed by single crystal X-ray analysis. During the catalytic activity, complexes 1 and 2 interestingly formed nanoparticles (NPs) (50–60 nm) in the presence of a base which was supported by FESEM and PXRD analysis and demonstrated efficient TH of various aromatic ketones providing good to excellent yields (∼90–100%) under mild reaction conditions which were authenticated by GC-MS/NMR/XPS analysis. Additionally, the formation of isopropylideneacetone established the in situ generation of acetone and suggests the formation of a metal hydride intermediate. Both the catalysts (1 and 2) showed strong selectivity exclusively towards aromatic ketones, while the corresponding aldehydes showed negligible conversions.


 Please wait while we load your content...
Please wait while we load your content...