Rhodium-catalyzed cascade C–H activation/annulation/1,6-acyl migration: direct construction of free N–H indoles under mild conditions†
Abstract
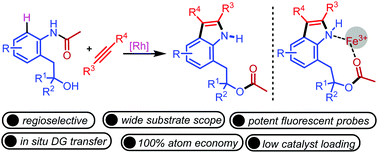
A Rh(III)-catalyzed C–H activation of acetanilides bearing β-hydroxy substituents with alkynes is described. The reaction proceeds via cascade C–H activation/annulation and 1,6-acyl migration to provide rapid access to diverse O-acylated N–H indoles in excellent yields. This catalytic method is regioselective for unsymmetrical alkynes and is compatible with primary, secondary, and tertiary compounds as well as chiral β-hydroxy compounds. This environmentally benign method has several advantages, such as cascade C–C/N–C/O–C bond formation, low catalyst loading, 100% atom economy, and high regioselectivity. Moreover, it requires acetone as a green solvent and involves in situ directing group transfer. Importantly, indoles bearing acyl and N–H coordination sites have been utilized as potent fluorescent probes for the selective sensing of Fe3+ ions.


 Please wait while we load your content...
Please wait while we load your content...