Construction of chiral Betti base precursors containing a congested quaternary stereogenic center via chiral phosphoric acid-catalyzed arylation of isoindolinone-derived ketimines†
Abstract
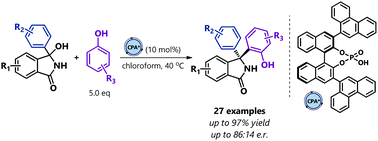
Synthesis of enantioenriched Betti base precursors containing a congested quaternary stereocenter is described. In a chiral phosphoric acid-catalyzed reaction, a series of in situ generated isoindolinone-derived ketimines and phenol derivatives yield products in good to excellent yields, and moderate enantioselectivities. Conversion of the obtained products into chiral Betti bases with the retention of the optical purity is demonstrated.


 Please wait while we load your content...
Please wait while we load your content...