Modulating defibrillation by tryptophan-mediated photo cleavage of disulfide bonds†
Abstract
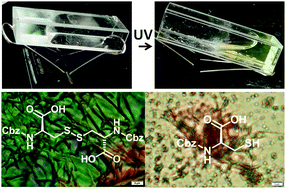
Tryptophan-mediated photo cleavage of disulfide bonds and the resulting defibrillation process have been demonstrated. A supramolecular fiber-like network structure arises due to the self-assembly of dibenzyloxycarbonyl-L-cystine by non-covalent interactions such as hydrogen bonding, π–π interactions and hydrophobic effects. The entangled fiber network encapsulates water and forms a hydrogel. The hydrogel is thixotropic in nature and responsive to external stimuli like heat and pH. However, on UV irradiation, Trp-mediated disulfide bond cleavage occurs, leading to defibrillation and a gel-to-sol transition. The fiber is amyloid-like and exhibited positive results in the Congo red assay. But the sol does not respond to the Congo red assay. Ellman's test further confirmed the photo-induced cystine to cysteine conversion. In the presence of Ellman's reagent, on UV exposure of the DZC hydrogel, a sharp absorption intensity at 412 nm occurs due to the reaction of newly generated thiolate anions from DZC with 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB).


 Please wait while we load your content...
Please wait while we load your content...