Impact of positive charge and ring-size on the interactions of calixarenes with DNA, RNA and nucleotides†
Abstract
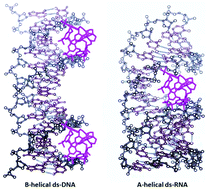
Comparison of various calix[6]arene and calix[4]arene derivatives revealed that only analogues bearing a permanent positive charge non-covalently bind to ds-DNA and ds-RNA, by insertion into a DNA minor groove or RNA major groove. Also, these cationic analogues revealed strong and highly selective charge-dependent stabilization of AT-DNA against thermal denaturation, the calix[6]arene trication being an order of magnitude more efficient than its calix[4]arene dicationic analogue. At variance to DNA/RNA selectivity for only cationic calixarenes, both neutral and cationic calixarenes bind nucleoside monophosphates with similar efficiency, by forming tweezer-like supramolecular complexes, with nucleobases inserted between aromatic pendant arms grafted to calixarene rims. Such nucleotide-calixarene complexes were monitored by an emission change of calixarene as a function of nucleobase insertion, at variance to DNA/RNA complexes in which calixarene is inserted into the polynucleotide groove, which does not change calixarene emission – stressing the importance of the ligand insertion within a calix-basket for fluorimetric sensing.


 Please wait while we load your content...
Please wait while we load your content...