Ag supported on alumina for the epoxidation of 1-hexene with molecular oxygen: the effect of Ag+/Ag0†
Abstract
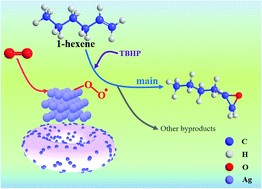
Linear terminal olefin epoxidation is an important oxidation reaction that provides valuable chemical intermediates. Heterogeneous catalytic epoxidation with O2 is a “dream reaction”, although it is still far from commercialization. In this study, bowl-shaped γ-Al2O3 (153 m2 g−1)-supported 1 wt% silver catalysts were obtained by the ammonia-evaporation method and subsequent calcination at 250–450 °C. Their catalytic liquid-phase epoxidation of 1-hexene with O2 performance was then studied. It was found that the initiator (TBHP) promotes selective oxidation and the 1,2-epoxyhexane is the major product (yield 6.08%), whereas excess dosage (0.8 mmol) and extra O2 partial pressure (0.5 MPa) led to an increase in further oxidation products, such as 1-hydroxyhexan-2-one. Considering the different chemical states of silver, the calcination temperature did not significantly affect the structural properties (specific surface area of ∼160 m2 g−1, pore volume of 0.37 cm3 g−1) or Ag particle size (10–20 nm), so the variation in the catalytic epoxidation performance was therefore attributed to the oxidized silver content. Further, it was speculated that oxidized silver readily activates the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and further dissociates and adsorbs oxygen to promote the selective oxidation of the reaction.
C bond and further dissociates and adsorbs oxygen to promote the selective oxidation of the reaction.


 Please wait while we load your content...
Please wait while we load your content...