Synthesis of bacteriochlorins bearing diverse β-substituents†
Abstract
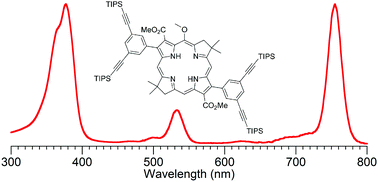
Synthetic bacteriochlorins, analogues of native bacteriochlorophylls, provide absorption in the near-infrared spectral region and hence are valuable for fundamental studies in photochemistry and applications ranging from solar energy conversion to photomedicine. The full realization of such opportunities hinges on access to synthetically malleable bacteriochlorins. Here, two established routes that rely on self-condensation of a dihydrodipyrrin-acetal or dihydrodipyrrin-carboxaldehyde have been exploited to prepare 6 bacteriochlorins (2 new, 4 known in improved fashion). Each bacteriochlorin contains a gem-dimethyl group in the reduced, pyrroline ring to ensure stability toward adventitious dehydrogenation. These and related synthetic bacteriochlorins have been derivatized to tailor the perimeter of the macrocycle with diverse groups, affording 9 additional new bacteriochlorins and 4 known bacteriochlorins. By direct installation and/or derivatization, the scope of attached entities in this collection includes alkyl, amino, aryl, bromo, carboethoxy, oxo, phenylethynyl, and pyridyl. The bacteriochlorins are suited for surface attachment, bioconjugation, water-solubilization, vibrational studies, and elaboration into multichromophore arrays. Altogether the synthesis of 25 new compounds (11 bacteriochlorins, 14 precursors) is described.


 Please wait while we load your content...
Please wait while we load your content...