Fluorinated tolane-based fluorophores bearing a branched flexible unit with aggregation-induced emission enhancement characteristics†
Abstract
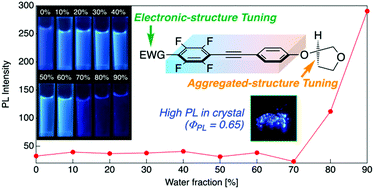
Small fluorescent molecules like tolanes are advantageous for broad applications, such as those in luminescence sensors and organic light-emitting devices. However, tolane derivatives are non-fluorescent in solution because of their immediate internal conversion from ππ* to dark πσ* excited states with a trans-bend structural shape. We focused on the development of tolane-based fluorescent molecules and found that the incorporation of fluorine atoms into one aromatic ring of tolane contributed to the emission of fluorescence owing to the electron density distribution and rigid molecular aggregated structure formed by intermolecular hydrogen bonding. Then, we focused on the molecular modulation, particularly the introduction of a branched structure, of a flexible unit that does not influence the electron density distribution; this could increase the interfacial distance between two tolanes to suppress non-radiative processes through energy transfer. Fluorinated tolanes were found to luminesce in dilute solutions with a relatively weak photoluminescence (PL) efficiency, whereas it was revealed that a cyclic flexible unit, such as a tetrahydrofuran ring, was the optimal structure for the flexible unit to exhibit intense PL in the crystalline state with high PL efficiency. Additionally, fluorinated tolane-based fluorophores bearing a cyclic flexible unit were found to exhibit aggregation-induced emission enhancement characteristics. The findings of this study offer a novel molecular design strategy to develop intense solid-state tolane-based luminophores.


 Please wait while we load your content...
Please wait while we load your content...