Dithiocarbamate modification of activated carbon for the efficient removal of Pb(ii), Cd(ii), and Cu(ii) from wastewater†
Abstract
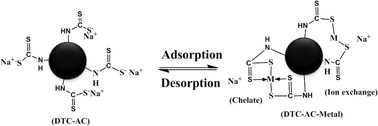
In this study, dithiocarbamate functionalized activated carbon (DTC-AC) was synthesized with nitric acid, tetraethylenepentamine (TEPA), and carbon disulfide (CS2). N2 adsorption–desorption technology, SEM, FTIR, and elemental analysis demonstrated the successful preparation of adsorption materials. Batch adsorption experiments were conducted to evaluate the influence of variable conditions on the adsorption behavior of Pb(II), Cu(II), and Cd(II) onto DTC-AC. Experimental data were fitted with adsorption kinetics models and isothermal models. The findings confirmed that DTC-AC exhibited a superior heavy metal adsorption capacity to oxidized AC (O-AC) and AC, and that pH played an important role in heavy metal removal by altering the surface charge. The adsorption kinetics study showed that the adsorption equilibrium was attained within 20 min, and the adsorption rate was dependent on both physisorption and chemisorption. The Langmuir model best described the adsorption performance of DTC-AC, and the maximal single metal uptake for Pb(II), Cu(II), and Cd(II) was 203.36, 53.13, and 102.89 mg g−1, respectively. Moreover, DTC-AC showed selective adsorption for Pb(II) in the ternary metal species system. Additionally, thermodynamic parameters showed that the adsorption process of heavy metals onto DTC-AC was spontaneous and endothermic. The heavy metal removal efficiency of DTC-AC remained above 85% after five consecutive adsorption–desorption cycles. This study showed that DTC-AC is an effective and reusable adsorbent with great potential for application in remediating heavy metal pollution.


 Please wait while we load your content...
Please wait while we load your content...