Diethyl phosphite-mediated switchable synthesis of bis(imidazoheterocycles) derived disulfanes and sulfanes using imidazoheterocycles and octasulfur†
Abstract
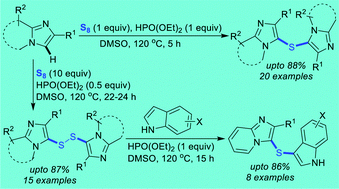
A practical and highly efficient oxidative dual C–H sulfenylation of imidazoheterocycles using odorless, inexpensive elemental sulfur in DMSO to synthesize sulfur-bridged imidazoheterocycles under metal-free conditions is reported. The amount of diethyl phosphite and sulfur powder most attractively permits a tunable synthesis of bis(imidazoheterocycle)disulfanes and bis(imidazoheterocycle)sulfanes in good to high yields. A comprehensive substrate scope with a broad range of functional group tolerance was realized, and the efficacy of the process was proved at gram-scale reactions. Next, the bis(imidazopyridine)disulfanes were smoothly reacted with various indoles under similar conditions to form the corresponding imidazo[1,2-a]pyridine-indole-derived thioethers in high yields. A plausible mechanism has been proposed based on the control experiments.


 Please wait while we load your content...
Please wait while we load your content...