Synthesis and biological evaluation of isatin oxime ether-tethered aryl 1H-1,2,3-triazoles as inhibitors of Mycobacterium tuberculosis†
Abstract
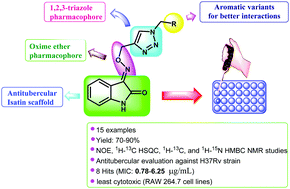
A series of fifteen novel isatin oxime ether-tethered aryl 1H-1,2,3-triazole hybrids 5a–o were designed and synthesized by employing Cu(I) catalyzed azide–alkyne [3+2] cycloaddition (CuAAC) between isatin oxime O-propargyl ether and aryl azides. The terminal alkyne moiety, isatin oxime O-propargyl ether 3, was synthesized by converting isatin 1 into isatin oxime 2 with subsequent O-propargylation. All the triazole hybrids are remarkably stable crystalline solids and were obtained in good yields. The compounds were well characterized by multinuclear NMR spectroscopy (1H, 13C and 19F), LCMS (ESI) and elemental analysis. The structure of compounds 3 and 5a was unambiguously established by means of multiplicity edited HSQC, NOE, 1H–13C HMBC, and 1H–15N HMBC NMR studies. The newly synthesized compounds have been screened for their in vitro antitubercular activity against M. tuberculosis H37Rv (ATCC 27294 strain). Among these compounds, eight compounds have shown good MIC (0.78–6.25 μg mL−1) values in comparison with the standard drugs. Furthermore, these potent compounds exhibited low in vitro cytotoxicity profiles against RAW 264.7 cell lines. The drug-likeness profile of the promising compounds 5d (MIC 0.78 μg mL−1), 5e (MIC 1.56 μg mL−1), 5h (MIC 1.56 μg mL−1), and 5i (MIC 3.125 μg mL−1) with selectivity index (SI) >10 evaluated by the SwissADME prediction web tool indicated the compliance of these compounds with drug-likeness rules/filters, which further shows the potential of the synthesized molecules as drug candidates.


 Please wait while we load your content...
Please wait while we load your content...