Ni(OH)2 Coated CoMn-layered double hydroxide nanowires as efficient water oxidation electrocatalysts†
Abstract
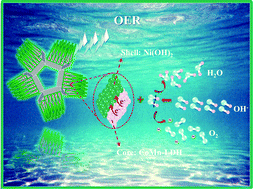
Core–shell nanowires of first-row transition metals are important members of nanostructured electrocatalysts for water oxidation owing to their superior electrocatalytic performance and tremendous promise as substitutes for noble-metal-based electrocatalysts. Recently, core–shell structures have attracted plenty of attention in the field of sustainable and affordable energy conversion and storage. In this research, we have prepared a hierarchical structure of nickel hydroxide (Ni(OH)2) coated on CoMn layered double hydroxide (CoMn-LDH) nanowires (labeled as Ni(OH)2@CoMn-LDH) as an oxygen evolution reaction (OER) electrocatalyst. This as-prepared Ni(OH)2@CoMn-LDH/NF electrode exhibits excellent oxygen evolution reaction (OER) performance with a lower overpotential of 250 and 341 mV at a current density of 30 and 100 mA cm−2 respectively (without iR-correction) along with a lower Tafel slope of 102 mV dec−1. In addition, Ni(OH)2@CoMn-LDH/NF remains stable for more than 25 h in 1 M KOH electrolyte. The outstanding OER behavior was ascribed to the ultrathin Ni(OH)2 coating deposited on conducting CoMn-LDH, which provides strong active sites with sufficient channels for electron transfer.


 Please wait while we load your content...
Please wait while we load your content...