3′-Amino modifications enhance the antifungal properties of N4-alkyl-5-methylcytidines for potential biocides†
Abstract
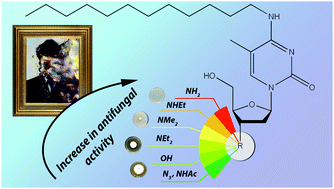
In order to develop a new generation of biocides with a wide spectrum of activities suppressing both bacteria and fungi, we have synthesized a representative library of novel 3′-modified derivatives of N4-alkyl-5-methyl-2′,3′-dideoxycytidines. The replacement of the 3′-hydroxyl group with amino, aminoethyl and dialkylamino groups significantly enhances the antifungal activity, most prominently towards the Aspergillus genus. Several new compounds were able to completely inhibit the growth of all the tested moulds (at 0.7 mM) isolated from works of art at the State Tretyakov Gallery, Moscow, Russia. Thus, such compounds are promising for use as biocides for protecting works of art from mould fungi. The synthesized compounds were also assessed for bacterial growth inhibition, demonstrating MIC values slightly above those of the currently used therapeutic drugs.


 Please wait while we load your content...
Please wait while we load your content...