One-pot synthesis of 3-substituted-3-hydroxyindolin-2-ones: three component coupling of N-protected isatin, aryne precursor and 1,3-cyclodione under metal-free conditions†
Abstract
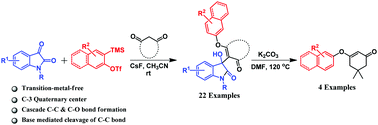
An aryne-based synthetic protocol has been developed for the synthesis of 3-substituted-3-hydroxy-indolin-2-ones. A wide variety of 3-hydroxyindolin-2-ones were synthesized in good yields under metal-free conditions via three component coupling of N-protected isatin, aryne precursor, and 1,3-cyclodione. One of our synthesized compounds has been unambiguously confirmed by single crystal XRD analysis. Further, treating the synthesized 3-hydroxyindolin-2-one derivatives with an inorganic base at high temperature leads to an interesting o-arylated product of 1,3-cyclohexandione.


 Please wait while we load your content...
Please wait while we load your content...