MoS2 oxidative etching caught in the act: formation of single (MoO3)n molecules†
Abstract
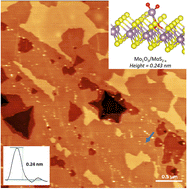
We report the presence of sub-nm MoOx clusters formed on basal planes of the 2H MoS2 crystals during thermal oxidative etching in air at a temperature of 370 °C. Using high resolution non-contact atomic force microscopy (AFM) we provide a histogram of their preferred heights. The AFM results combined with density functional theory (DFT) simulations show remarkably well that the MoOx clusters are predominantly single MoO3 molecules and their dimers at the sulfur vacancies. Additional Raman spectroscopy, and energy and wavelength dispersive X-ray spectroscopies as well as Kelvin probe AFM investigations confirmed the presence of the MoO3/MoOx species covering the MoS2 surface only sparsely. The X-ray absorption near edge spectroscopy data confirm the MoO3 stoichiometry. Taken together, our results show that oxidative etching and removal of Mo atoms at the atomic level follow predominantly via formation of single MoO3 molecules. Such findings confirm the previously only proposed oxidative etching stoichiometry.


 Please wait while we load your content...
Please wait while we load your content...