A versatile strategy to construct free-standing multi-furcated vessels and a complicated vascular network in heterogeneous porous scaffolds via combination of 3D printing and stimuli-responsive hydrogels†
Abstract
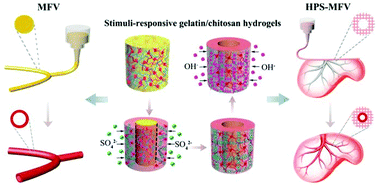
Mimicking complex structures of natural blood vessels and constructing vascular networks in tissue engineering scaffolds are still challenging now. Herein we demonstrate a new and versatile strategy to fabricate free-standing multi-furcated vessels and complicated vascular networks in heterogeneous porous scaffolds by integrating stimuli-responsive hydrogels and 3D printing technology. Through the sol–gel transition of temperature-responsive gelatin and conversion between two physical crosslinking networks of pH-responsive chitosan (i.e., electrostatic network between protonated chitosan and sulfate ion, crystalline network of neutral chitosan), physiologically-stable gelatin/chitosan hydrogel tubes can be constructed. While stimuli-responsive hydrogels confer the formation mechanism of the hydrogel tube, 3D printing confers the feasibility to create a multi-furcated structure and interconnected network in various heterogeneous porous scaffolds. As a consequence, biomimetic multi-furcated vessels (MFVs) and heterogeneous porous scaffolds containing multi-furcated vessels (HPS-MFVs) can be constructed precisely. Our data further confirm that the artificial blood vessel (gelatin/chitosan hydrogel tube) shows good physiological stability, mechanical strength, semi-permeability, hemocompatibility, cytocompatibility and low in vivo inflammatory response. Co-culture of hepatocyte (L02 cells) and human umbilical vein endothelial cells (HUVECs) in HPS-MFVs indicates the successful construction of a liver model. We believe that our method offers a simple and easy-going way to achieve robust fabrication of free-standing multi-furcated blood vessels and prevascularization of porous scaffolds for tissue engineering and regenerative medicine.


 Please wait while we load your content...
Please wait while we load your content...