Design and synthesis of yellow- to red-emitting gold(iii) complexes containing isomeric thienopyridine and thienoquinoline moieties and their applications in operationally stable organic light-emitting devices†‡
Abstract
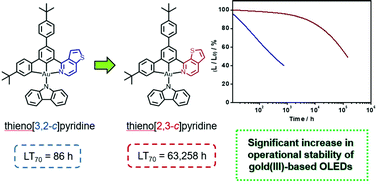
A new class of yellow- to red-emitting carbazolylgold(III) complexes containing isomeric thienopyridine or thienoquinoline moieties in the cyclometalating ligand has been designed and synthesized, which showed high photoluminescence quantum yields of over 80% in solid-state thin films. The isomeric effect and extended π-conjugation of the N-heterocycles have been found to remarkably perturb the photophysical, electrochemical and electroluminescence properties of the gold(III) complexes. In particular, the operational lifetimes of organic light-emitting devices based on that incorporated with thieno[2,3-c]pyridine are almost three orders of magnitude longer than that incorporated with thieno[3,2-c]pyridine. This has led to long device operational stability with a LT70 value of up to 63 200 h at a luminance of 100 cd m−2 and a long half-lifetime of 206 800 h, as well as maximum external quantum efficiencies of up to 8.6% and 14.5% in the solution-processed and vacuum-deposited devices, respectively. This work provides insights into the development of robust and highly luminescent gold(III) complexes and the identification of stable molecular motifs for designing efficient emitters.
- This article is part of the themed collections: Celebrating International Women’s Day: Women in Materials Science and Special issue in honour of Seth Marder


 Please wait while we load your content...
Please wait while we load your content...