Aggregation-induced enhanced fluorescence by hydrogen bonding in π-conjugated tricarbocycles with a CF2CF2-containing cyclohexa-1,3-diene skeleton†
Abstract
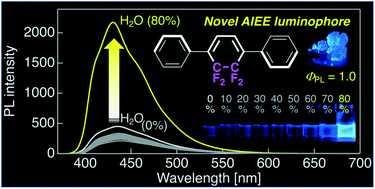
On the basis of the understanding of CF2CF2-containing organic molecules, π-conjugated tricarbocycles with a CF2CF2-containing cyclohexa-1,3-diene skeleton have recently been prepared from commercially available 4-bromo-3,3,4,4-tetrafluorobut-1-ene in eight steps. Solid-state luminescent molecules have been actively developed in recent years, but the molecular design of efficient luminophores has been limited. Based on the knowledge that the fluorine atoms in molecular structures are responsible for the enhanced luminescence efficiency through H⋯F hydrogen bonds, the purpose of this study was to clarify the applicability of π-conjugated tricarbocycles with a CF2CF2-containing cyclohexa-1,3-diene skeleton as a new molecular design for solid-state luminophores. The π-conjugated tricarbocycles showed fluorescence in a dilute solution but had a low fluorescence efficiency. However, adding 80% water to a tetrahydrofuran solution dramatically increased the fluorescence efficiency. In the crystalline state, the tricarbocycles showed blue or green fluorescence with a very high fluorescence efficiency, owing to the formation of intermolecular C⋯F hydrogen bonds. This proved that tricyclic π-conjugated molecules have aggregate-induced emission enhancement properties. These results indicate that π-conjugated tricarbocycles with a CF2CF2-containing cyclohexa-1,3-diene skeleton have promising solid-state luminescence performance, paving the way for the development of new solid-state luminophores, such as organic light-emitting diodes or lighting devices.


 Please wait while we load your content...
Please wait while we load your content...