Rational design of nickel–borane complexes for methane activation and functionalization†
Abstract
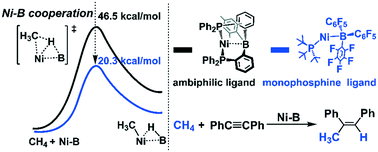
Efficient utilization of earth-abundant methane provides a sustainable protocol to solve both the greenhouse effect and the increasing energy crisis. Here, we rationally designed active nickel–borane (Ni–B) complexes for efficient methane activation and functionalization through DFT calculations. The reaction occurs through the cooperative functions of the Ni center and the borane moiety, where the mono-phosphine ligand supported Ni–B complexes (1BPh and 1BCF) are more active than the ambiphilic ligand supported 1Ni–B. 1BCF is more active than 1BPh because the electron-withdrawing substituents on the borane moiety enhance the charge transfer from the σ-bonding orbital of CH4 to the LUMO of the Ni–B moiety. Next, a hydromethylation catalytic cycle mediated by 1BCF was constructed by employing diphenylacetylene as the substrate. The reaction is initiated by the successive association of diphenylacetylene and CH4 with 1BCF to form a stable complex 3. Starting from 3, the CH4 activation occurs with Gibbs energy barrier (ΔG°≠) and Gibbs reaction energy (ΔG°) values of 20.3 and 9.1 kcal mol−1, respectively. Then, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond is inserted into the Ni–methyl bond (the rate-determining step) followed by a reductive elimination step to produce the final product cis-1,2-diphenylpropene to complete the catalytic cycle. The ΔG°≠/ΔG° values of the insertion and reductive elimination steps are 26.9/−13.4 and 13.4/−7.3 kcal mol−1, respectively, suggesting that this reaction would be achieved under mild conditions.
C triple bond is inserted into the Ni–methyl bond (the rate-determining step) followed by a reductive elimination step to produce the final product cis-1,2-diphenylpropene to complete the catalytic cycle. The ΔG°≠/ΔG° values of the insertion and reductive elimination steps are 26.9/−13.4 and 13.4/−7.3 kcal mol−1, respectively, suggesting that this reaction would be achieved under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...