Exploration of piperidine 3D fragment chemical space: synthesis and 3D shape analysis of fragments derived from 20 regio- and diastereoisomers of methyl substituted pipecolinates†
Abstract
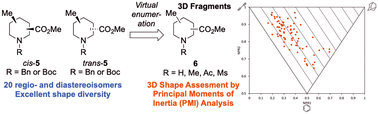
Fragment-based drug discovery is now widely adopted for lead generation in the pharmaceutical industry. However, fragment screening collections are often predominantly populated with flat, 2D molecules. Herein, we report the synthesis of piperidine-based 3D fragment building blocks – 20 regio- and diastereoisomers of methyl substituted pipecolinates using simple and general synthetic methods. cis-Piperidines, accessed through a pyridine hydrogenation were transformed into their trans-diastereoisomers using conformational control and unified reaction conditions. Additionally, diastereoselective lithiation/trapping was utilised to access trans-piperidines. Analysis of a virtual library of fragments derived from the 20 cis- and trans-disubstituted piperidines showed that it consisted of 3D molecules with suitable molecular properties to be used in fragment-based drug discovery programs.
- This article is part of the themed collection: Fragment-based drug discovery


 Please wait while we load your content...
Please wait while we load your content...