Inhibition of MARK4 by serotonin as an attractive therapeutic approach to combat Alzheimer's disease and neuroinflammation†
Abstract
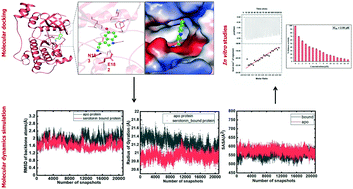
Mitogen-activated protein kinases (MAPKs) govern various cellular programs and crucial intermediate pathways in signaling. Microtubule affinity-regulating kinase 4 (MARK4) is a part of the kinase family recognized for actively phosphorylating neural microtubule-associated proteins (MAPs) like MAP2, MAP4 and most importantly, tau. The Ser/Thr kinase MARK4 overexpression is associated with various life-threatening conditions such as neurodegenerative disorders, diabetic neuropathy, and cancer. Functionally, MARK4 is correlated with many important signaling cascades and transcription factors contributing to neurodegeneration and cancer onset and progression. Serotonin is a key molecule associated with regulating mood, stress, and various behavioral aspects. Low serotonin levels promote the progression of neurological and psychotic disorders, which is also a consequence of tau accumulation. MARK4 being a major contributor to phosphorylating tau, leading to its accumulation, and contributing to tauopathy, is targeted for inhibition by serotonin. The study deals with the inhibition of MARK4 by serotonin using combined computational and experimental studies. The results presented in this paper provide strong evidence for the direct physical binding of serotonin to recombinant MARK4 and subsequent inhibition of its kinase activity. In addition, we have performed molecular docking, followed by 100 ns MD simulations of MARK4 in the presence of serotonin, to estimate the stability of the protein–ligand complex. Since MARK4 is a potential drug target and can be exploited for drug design and discovery for cancer and neurodegenerative disorders, the results presented here are of interest and may be further exploited for Alzheimer's disease (AD) and other neurodegenerative diseases.


 Please wait while we load your content...
Please wait while we load your content...