Design and evaluation of poly-nitrogenous adjuvants capable of potentiating antibiotics in Gram-negative bacteria†
Abstract
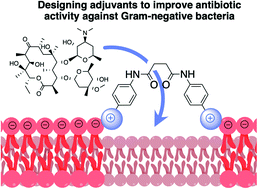
Antibiotic resistance has been a growing public health crisis since the 1980s. Therefore, it is essential not only to continue to develop novel antibiotics but also to develop new methods for overcoming resistance mechanisms in pathogenic bacteria so antibiotics can be reactivated towards these resistant strains. One common cause of antibiotic resistance in Gram-negative bacteria is reduced permeability of the tightly packed, negatively charged lipopolysaccharide outer membrane (OM), which dramatically reduces or even prevents antibiotic accumulation within the cell. Adjuvants that promote passive diffusion through the OM, including phenylalanine-arginine-β-naphthylamide, tobramycin, and pentamidine, have proven useful in potentiating antibiotics against Gram-negative bacteria. Structural evaluation of these adjuvants, which all include multiple nitrogenous groups, indicates that the entry rules developed for improving antibiotic accumulation in Escherichia coli (EC), could also be used to guide adjuvant development. To this end, a series of structurally simple poly-nitrogenous diphenylsuccinamide compounds have been prepared and evaluated for their ability to potentiate a panel of classic antibiotics in wild-type EC and Pseudomonas aeruginosa (PA). Modest adjuvant activity was observed for all compounds surveyed when co-administered with known antibiotics to inhibit either wild-type EC or PA, and all were able to accumulate in both EC and PA.


 Please wait while we load your content...
Please wait while we load your content...