Synthesis of SrTiO3 and Al-doped SrTiO3via the deep eutectic solvent route†
Abstract
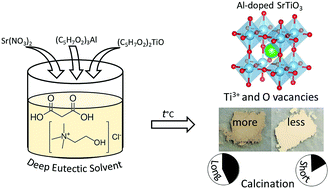
SrTiO3 and aluminum-doped SrTiO3 are synthesized by calcination of metal salts dissolved in a deep eutectic solvent (DES) without any post-synthesis treatment. The DES used is the eutectic mixture of choline chloride (hydrogen bond acceptor) and malonic acid (hydrogen bond donor). Titanium(IV) oxide bis(2,4-pentanedionate) is utilized as the non-volatile, easy-to-handle, DES-soluble titanium precursor. The ammonia gas evolved during the calcination process provides a reducing atmosphere, resulting in the formation of Ti3+ and oxygen vacancies within the SrTiO3 matrix. According to UV-Vis spectroscopy and X-ray photoelectron spectroscopy, the amount of Ti3+ species and oxygen vacancies (VO) in the synthesized perovskite can be tuned by varying the duration of the calcination process and by adding Al3+ dopants. Solid state 27Al NMR spectroscopy and powder X-ray diffraction confirm the doping of aluminum into the octahedral site of the perovskite structure. Surface photovoltage spectroscopy confirms that Al3+ dopants can eliminate Ti3+ defects in Al-doped SrTiO3. Ultraviolet illumination experiments in water and aqueous methanol show that SrTiO3 and aluminum-doped SrTiO3, after modification with RhxCr2−xO3 or Pt co-catalysts, evolve small amounts of H2 (EQE of 0.0113–0.0173% at 375 nm) with only traces of O2 detected. The lack of photocatalytic activity is attributed to rapid electron-hole recombination in the oxygen vacancy-rich materials and to the lack of crystal facets that could aid charge separation.
- This article is part of the themed collection: Popular Advances


 Please wait while we load your content...
Please wait while we load your content...