Tuning ionic conductivity to enable all-climate solid-state Li–S batteries with superior performances†
Abstract
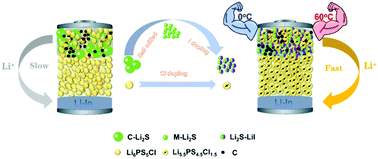
Low Li-ion mobility of the cathode mixture is one of the major obstacles for Li2S-based solid-state Li–S achieving excellent electrochemical performances. The poor Li-ion conductivity is due to the intrinsic insulation of Li2S and the low Li-ion mitigation across the Li2S/solid electrolyte interface. Here, we propose the correlation between the increased Li-ion conductivity of the cathode mixture and electrochemical performances of solid-state batteries using Li2S as an active material. Replacing Li6PS5Cl with a superior conductive Li5.5PS4.5Cl1.5 solid electrolyte increases the interfacial ionic mobility and reduces the solid/solid resistance, resulting in higher discharge capacities and better cycling performances. In addition, the Li-ion conductivity of Li2S is enhanced by reducing the particle sizes using high-rotation milling, and a further improvement is achieved by mixing the obtained milled Li2S with LiI. The 3Li2S–LiI cathode mixture with high room temperature ionic conductivity and a comparable Li2S loading amount is chosen as the cathode and combined with the Li5.5PS4.5Cl1.5 solid electrolyte to fabricate solid-state Li–S batteries. The assembled battery displays excellent electrochemical performances at different operating temperatures. Our findings in this work could help to promote the development of Li2S-based solid-state Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...