Chalcogen-substituted PCBM derivatives as ternary components in PM6:Y6 solar cells†
Abstract
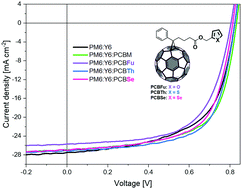
Ternary organic solar cells (TOSCs) are a promising approach to enhance the power conversion efficiency in organic-based solar cells. The combination of fullerene and non-fullerene acceptors is employed to optimize the light absorption and phase separation for better charge dissociation and collection. Herein, we describe the synthesis and characterization of three chalcogen substituted PCBM derivatives with a 5-membered aromatic ring linked to the methyl ester position with the objective to study the relationship between interphase separation and power conversion efficiency. Therefore, the effects of furan, thiophene and selenophene in the PCBM derivatives (PCBFu, PCBTh and PCBSe, respectively) are investigated on the photovoltaic performance of ternary organic solar cells based on PM6:Y6. We observed that the addition of the PCBTh and PCBSe derivatives increases the short circuit current density and the fill factor pointing to the suppression of charge recombination. In addition, surface and thermal analysis confirms that the morphology is optimized in both cases, which implies that organized thin film nanomorphology is key for the suppression of carrier losses. The TOSC with PCBTh exhibited the highest power conversion efficiency among all the devices reaching 14.6%.
- This article is part of the themed collection: Organic Electronics – Ecofriendly and/or sustainable materials, processes, devices, and applications


 Please wait while we load your content...
Please wait while we load your content...