A preliminary study of rapid measurements of aqueous 210Po by accelerator mass spectrometry
Abstract
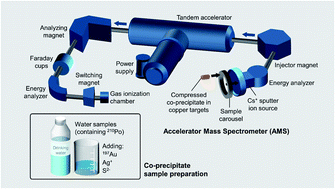
Polonium-210 (210Po) is a toxic radioisotope naturally present in freshwater and groundwater that threatens the safety of drinking water supply. Designed for rapid analyses, a new procedure was tested for measuring aqueous 210Po by accelerator mass spectrometry (AMS). Since the longer-lived polonium isotopes 208Po and 209Po suffer from atomic isobaric interferences, we normalized the 210Po ion beam to a beam of 197Au as the reference isotope. 210Po and 197Au solutions were co-precipitated with Ag+ and S2− to form the stable compound Ag2S. This method homogeneously precipitated these two isotopes of different elements and provided good electrical and thermal conductivity when the dried solid was pressed into a target for use in a Cs+ sputter ion source. Beams of atomic Po− were charge changed, and the Po3+ ion was used for subsequent mass and energy analysis. Ion beams of ≥0.20 counts per second per femtogram of 210Po in a target were produced. The measured and prepared 210Po/197Au ratios showed a good linear response when the quantity of 210Po was prepared in a dilution series. With a counting time of less than 1.5 hours per target, the procedure indicated a 210Po detection limit of 20 mBq (0.12 fg), supporting the next phase of development with environmental aqueous samples.


 Please wait while we load your content...
Please wait while we load your content...