Electrocatalytic synthesis: an environmentally benign alternative for radical-mediated aryl/alkenyl C(sp2)–C(sp3) cross-coupling reactions
Abstract
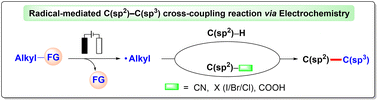
C(sp2)–C(sp3) bonds comprise the fundamental skeletons of many organic molecules, making their construction one of the most important transformations in synthetic chemistry. Over the past decades, radical-based C(sp2)–C(sp3) cross-coupling reactions have emerged as a straightforward and powerful tool to rapidly increase molecular complexity for numerous applications in biomedical science, chemistry, and materials science. Electrochemistry is regarded as a synthetically appealing alternative to drive redox reactions in a selective and environmentally benign manner. With the flourishing development of organic electrosynthesis, remarkable achievements have been witnessed in radical-mediated aryl/alkenyl C(sp2)–C(sp3) cross-coupling reactions for the synthesis of diverse alkylated (hetero)arenes and 1,2-disubstituted alkenes. In these reactions, the generation of alkyl radicals and their orderly transformation are the keys to success. This review comprehensively summarizes recent progress in electrochemically initiated radical-mediated C(sp2)–C(sp3) cross-coupling reactions, emphasizing the key electrochemically induced step for the generation of alkyl radical species, the scope and limitations of the C(sp2) and C(sp3) coupling partners, and the underlying reaction mechanisms.
- This article is part of the themed collection: Green Chemistry Reviews


 Please wait while we load your content...
Please wait while we load your content...