Electrochemical Minisci reaction via HAT-driven α-C(sp3)–H functionalization of alcohols†
Abstract
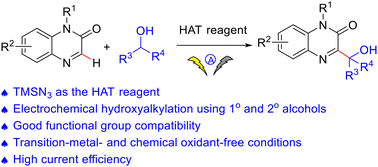
An efficient electrochemical Minisci reaction to access 3-hydroxyalkylquinoxalin-2(1H)-ones involving hydrogen-atom transfer (HAT) driven α-C(sp3)–H functionalization of alcohols was achieved. Transition metal- and chemical oxidant-free conditions were the attractive synthetic features. The hydrogen atom transfer agent was hydrazoic acid generated from TMSN3 in this transformation. Primary or secondary alcohols and a wide range of quinoxalinones were found to be compatible, providing the corresponding 3-hydroxyalkylquinoxalin-2(1H)-ones in good yields.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...